Laser Marking Machines for Medical Devices & Surgical Instruments / Equipments
Possibilities & Opportunities of Laser Marking in Medical Industry
Laser Annealing
Micro Machining
Precision Machining

Part Traceability & UDI
Medical Industry is one of the most high-risk product manufacturers. From ranging to wheel chair to the stent, all the products are directly related to health of the patient. Hence, error while manufacturing of these products or a minor defect could prove to be very costly to the life. Counterfeiting! Is the problem worst hit by genuine medical OEMs, this problem not only causes a lot of money to the manufacturer; but too have risked lives of millions. To deal with this dilemma one of the key solutions is traceability of the medical equipment.
Laser Marking on Surgical Scissor
Laser marking of UDI Codes on Stainless Steel Instruments
Annealing of the stainless steel done in accordance to GS1 standards with DMC and HRC. The surface of the steel is charred only to a certain depth in few microns to comply with the medical safety standard marking. These marks though permanent are also corrosion resistive. Apart from fulfilling UDI traceability requirement; direct part marking can also be helpful in healthcare organizations to manage their inventory and can be linked with patient ID as code in internal servers whereby the information could serve to connect patient surgery history with all the instruments used on them. DMC code is marked in respect to the guidelines defined by ISO-16022, which is an international standard for "Data Matrix Bar-code Symbology Specification”
Features
- Medical Device: Surgical Scissor
- Material: SS316 and SS 410
- Laser Machining Type: Annealing UDI
- Marked By: 20W DE- HF Fiber Laser
- Marking: Logo and 2D Data Matrix
- DMC Size: 2.71 mmx 2.71 mm Avg
- Marking Time: 5 sec
Preferred Machine

UDI Marking: Surgical Clips
Direct Code Marking on Titanium Instruments
Grade 1 titanium is one of the most used titanium grades in health care industries for manufacturing surgical instruments. Due to its superior form ability and corrosion resistive properties they are used to produce most complicated tiny surgical implants and instruments. The vessel clips are marked with UDI compliant codes and manufacturer logos in accordance to DPM guidelines defined in the standard ISO/IEC 24720. The main advantage of direct part marking is to avoid any chances of felling off the printed labels inside patients body while operating. These marks could easily withstand bodily plasma and high temperature.
Features
- Medical Device: Surgical Clips
- Material: Grade 1 Titanium
- Laser Machining Type: Ablation UDI Mark
- Marked By: 20W DE- HF Fiber Laser
- Marking: 2D Data Matrix
- DMC Size: 2.71 mm x 2.71 mm Avg
- Marking Time: 5 sec
Preferred Machine

Laser Marking on LDPE In-vitro Diagnostics Packaging, Nylon In-vitro Diagnostics & HDPE Bottles
Medicinal packaging comes with licence No., production information of company such as Lot No., Mfg. date & expiry dates. These data while manufacturing should be intact and must remain unaltered to avoid catfishing, removable data could prove to be fatal for the consumers. Hence, while packaging these data should be placed on the packet itself with laser marking either they belong to LDPE make or HDPE. We at Markolaser provide best of the solutions to pharmaceutical industries to place the information without use of detachable labels. HDPE in-vitro diagnostics are also clearly marked with scale to analyse the blood samples.
Features
- Medical Device: HDPE Bottles
- Material: Font marking
- Laser Machining Type: Annealing UDI
- Marked By: 20 W Normal Fiber Laser
- Marking: Logo and 2D Data Matrix
- DMC Size: 2.71 mm x 2.71 mm Avg
- Marking Time: 2s approx
Preferred Machine

Laser Marking on Medical Equipment Hip Balls
Micro-Data matrix code are real challenge to mark as well as read perfectly, due to space restrictions in conical & cylindrical bone screws made of titanium alloys which have maximum diameter of 1-2 mm; it is difficult to find flat spaces to mark. With our fine beam lasers, it is still possible to mark readable codes of size 0.4mm and indeed contrasting for total traceability of smaller implants.
Features
- Medical Device: Hip Balls
- Material: Chromium Vanadium
- Laser Machining Type: Black Marking
- Marked By: Fiber Laser
- Marking: Logo and 2D Data Matrix
Preferred Machine

Datamatrix Marking on Pipette, Burete, Flasks, Cylindrical Measurement
Features
- Medical Device: Pipette, Burete, Flasks, Cylindrical Measurement
- Material: Glass Borosilicate
- Laser Machining Type: Emulsion removal DMC
- Marked By: 20W Normal
- Marking: Logo and 2D Data Matrix
Preferred Machine

Branding & Seriallization on Tooth Crowning
Features
- Medical Device: Tooth crowning
- Material: Ceramic
- Laser Machining Type: Traceability Fonts (Serial No.), Black
- Marked By: Fiber Laser
- Marking: Logo and 2D Data Matrix
- DMC Size: 2.71 mm x 2.71 mm Avg
All the implants are marked by laser as these marks are biocompatible to bodily fluids and remains intact for several year, hence undetachable marks remains on the surface and is resistant toward the germs. Easy track and trace can be achieved. Laser markings are permanent and are abrasion, heat and acid resistant.
Preferred Machine

Laser Engraving on Conical or Cylindrical Dental Jaw Screw
Features
- Medical Device: Conical or Cylindrical Dental Jaw Screw
- Material: Titanium Alloy
- Laser Machining Type: Small DMC 0.6x0.6 mm, Annealing
- Marked By: 20W DE HF
- Marking: Logo and 2D Data Matrix
Preferred Machine

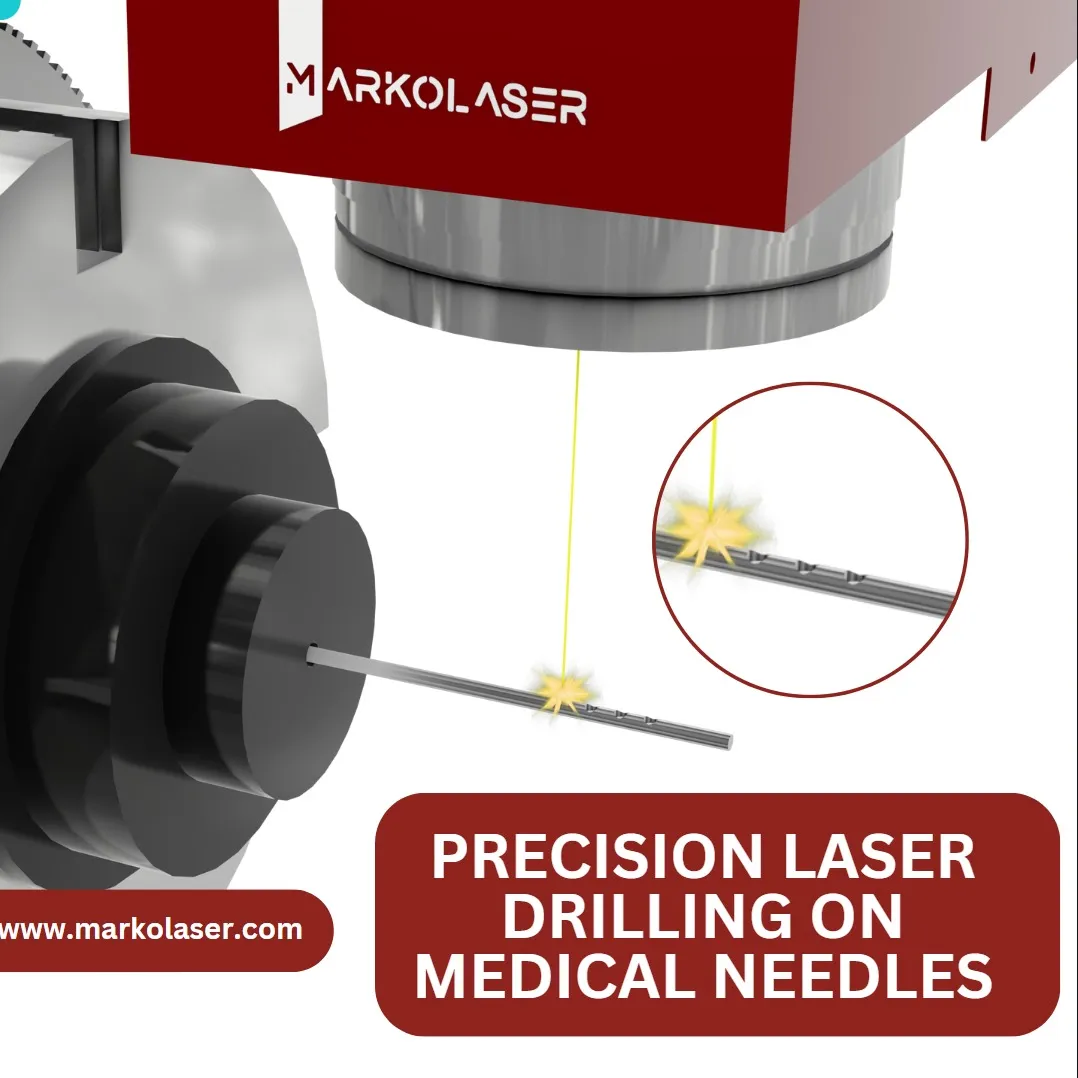
Precision Laser Drilling on Medical Needles
Features
- Medical Device: Medical Needles
- Material: Stainless steel
Medical needles serve multiple functions within the healthcare industry, including the injection and delivery of drugs, vitamins, antibiotics, and electrolytes into the body. They are used for both intramuscular and intravenous medication delivery. Additionally, needles are essential for procedures such as blood withdrawal. Recently, Markolaser was entrusted with a project involving laser drilling of holes with varying diameters on medical needles, based on provided specifications and artwork.
The Challenge
Laser marking on a lacquered surface presents unique challenges, particularly when trying to achieve clear, black, and visible QR codes. The lacquer's reflective properties can affect the laser's marking accuracy, requiring precise adjustments to ensure the desired effect
Preferred Machine
UDI Marking:
Unique device identification (UDI) is a series of numeric as well as alphanumeric characters
created through internationally accepted
device identification and coding standards and thus allowing unambiguous identification of
specific devices available in
marketSeptember 2013, Under FDASIA Act of 2012 amendment sec. 519(f), The U.S. Food and
Drugs Authority (F.D.A.) published
regulations for establishing UDI systems and came into law in year 2014 for the medical
devices to be marketed in U.S.
They have directed for standardized labelling to completely enable global identification and
tracking of medical devices.Though
these regulations are applied for medical products that are produced or imported in U.S. but
European Union and Countries like
Brazil, China, Australia and India are also planning to implement similar global standards
in their health industry and have
already prepared frameworks to do so. European commission is responsible for the
implementation of UDI regulation in E.U.
21 USC 321(h) defined the devices subjected to requirement of UDI rules and classified
medical products into three classes according
to their potential of causing harm to patient and functionality. For example, low risk
products such as bandages and
gloves are classified in class 1 devices whereas class 3 involves complex medical products
such as pacemakers, knee prosthesis
which have higher risk to human life if they malfunctioned after being implanted. Class 2
products usually involves surgical
instruments and moderate risk metal products for general hospital use. So according to UDI
norms these products are to be
marked according to their class. Some products need to be directly part marked, whereas some
can be identifified with UDI
marked on labels and packaging. These exceptions depend on various factors such as available
area of marking, either product
reprocessed or sterilized, whether device an implant etc. Mostly class 2 devices which
undergoes autoclaving cycles are needed
to be directly part marked. The rules and regulation could be found in e-CFR site under
section 21 CFR 801.45 (d).
click link: t.ly/l7Yg7
The UDI System Aims for:
- Increased safety of patients
- Authentic medical products
- Efficient product recall
- Better logistic and delivery process
- Better documentation of product in supply chain
- Prevention of incidents
- Reducing medical errors
As Per 21 CFR 801.40 the UDI on Medical Devices
Must be Represented
in Two Forms:
Machine readable code (linear bar code or 2D data matrix)- AIDC. Human readable plain text (alpha-numeric characters)- HRI. The UDI code must be issued by FDA issuing agencies and must follow ISO/IEC 15459- 2, -4, -6 standards. The AIDC format should follow the ISO/IEC 16022 and their print quality conforming to ISO/IEC 15415, 15416 & TR 29158.
The UDI Code Must Consist of Two Parts

Production Identifier (P.I.)

Device Identifier (D.I.)
UDI Database
The labeller and manufacturer of medical products with the UDI labelling must submit the labelling/marking information into a central database of FDA called The Global Unique Device Identification Database (GUDID- also pronounced as “Good ID”) containing key device identification information to adequately identify devices marketed in U.S. The GUDID consist of DI only; PI are not submitted or stored in GUDID. The GUDID data is linked globally, which helps to identify the device all round the world.FDA has created a portal namely "AccessGUDID", to make device identification information available for everyone involved in the health supply chain; even the patients can access the information on the online portal. https://accessgudid.nlm.nih.gov/
Characteristics of Direct Part Marking (DPM)
The required features of the direct part marking on medical
instruments are:The mark should be permanent, traceable
& error- free. Marks should be high in contrast, and readable
from all the angles. Marking should be resistant towards
corrosion and repeated sterilization cycle. DPM should not
affect the actual functionality of the device and remain passivated
Challenges Faced in DPM of UDI
One of the major problems faced for DPM of medical instruments such as surgical scissors, needles etc. which are mostly made up of steels, titanium; is the repeated sterilization cycle and resistance towards corrosion which thus affect their readability with passage of time, affecting the traceability which is contrasting to the idea of UDI. Also, poor positioning of marks, low contrast marks and wrong formatting is some of the major concerns for supplier chain and manufacturers. Hence, proper marking solutions are needed for manufactures to direct part mark their products to comply with the strict UDI regulations.
Laser Marking on the Surgical Instrument and UDI Traceability Laser
Surgical Instruments Such as :
Scissors, Forceps, Clamps, Needle Holders, Knives, Blades, Retractors, and Fiber Optic instruments & require serialization or traceability. Metals and plastic are used to manufacture these instruments. All these surgical tool manufacturers can benefit from Markolaser's med laser marker, which is developed and designed in accordance with UDI, MDR, and FDA standards and regulations.
Laser Marking Machine for Branding and UDI Data Matrix Unique Code Marking on Medical Peek and Orthopedic or Trauma
Surgical Instruments Such as :
Scissors, Forceps, Clamps, Needle Holders, Knives, Blades, Retractors, and Fiber Optic instruments & require serialization or traceability. Metals and plastic are used to manufacture these instruments. All these surgical tool manufacturers can benefit from Markolaser's med laser marker, which is developed and designed in accordance with UDI, MDR, and FDA standards and regulations.
Surgical Instruments:
Surgical instruments are mostly made up of steel, titanium, ceramics or synthetic resins. The most common ones are SS- 304, 316L, 410, 430. Due to their improved mechanical properties and stability they are used for surgical device, the laser marking is best used method for marking visible UDI marks as paper labelling could prove harmful; as there is a chance of falling off the labels while sterilization or during surgery in patient’s body. Why using laser for UDI DPM ? Why not other processes feasible ?
| Marking- Characteristics | Laser | Ink-Jet | Dot Peen | Chemical Etching |
|---|---|---|---|---|
| Readability | Good quality with high contrast; good machine-readability | Lower quality marks with low contrast | Poor mark resolution with low contrast | Good quality readable marks with high contrast |
| Erosion Resistance | Full proof resistance toward erosion | No resistance towards erosio | Resistive towards erosion | Erosion could be there for component |
| Sterilization Resistance | Resistive towards repetitive sterilization | Non-permanent marks containing chemical | Resistive towards sterilization | Risk of losing marking is there |
| Passivation Resistance | Resistive to passivation process | Resistive to passivati | Not too resistive to passivation | Resistive but risk is there (test needed) |
| Marking Size | Perfect marking obtained and size adjustable | Limited marking size obtained | Limited marking size | Compact codes cannot be printed |
Laser Marking on Medical Devices & its Advantages:
Laser marking has no alternate in permanent marking when it comes for medical devices that goes under repeated passivation. Mostly medical devices and surgical tools are made of stainless steel 304-316 or titanium. These materials consist of chromium oxide (corrosion-resistant layer) on its surface. A laser mark visually contrasts with the natural color of the alloy to make the mark visible – the purpose of the mark. The style of mark required by the medical device industry is called a "dark or annealed mark". This mark does not remove any material from the part and so avoids any potential for any contamination to collect. Standard fiber- lasers or picosecond laser can produce such type of dark marks. These dark mark from lasers are desirable as they are visible from all the angles. Laser marks are also precise and passivation resistance.

UDI Marking with Markolaser:
As already known medical industry specifications are quite strict and have to be precise, so is important the traceability of the product and thus the marking needs to perfect. We at MARKOLASER® provides complete solution for direct part marking with a single source laser and software module specially designed for generating high contrast, corrosion resistant marking at any kind of metallic surfaces. With our MARKOMARK software along with our high-speed nanosecond lasers generating accurate and stable black oxidation data matrix marks over repeated time; required for safe traceability of medical instruments. Also, our laser machine is hassle -free with short processing time and produces less fume thus the procedure remains contact free and hygienic, giving “cradle to grave” marking solution of complex 2D and linear bar codes. To comply with the strict specification of standardization in medical industry our research team follow these standards for marking and testing of samples for legitimacy of reading: < Document Related to Direct Part Marking (DPM) of surgical Instruments according to GS1 standards and following Japan association of medical devices industries (JAMDI) guidelines to comply with global UDI system of traceability. Using ISO standards of DPM quality guidelines (29158) for laser black marking on steel instruments. Corrosion testing of marked area complying with ISO (9227) and ASTM (F 1089) standards of corrosion test. Our research team have studied results on short pulse fiber laser marked 2D data matrix on SS 304 (passivated) surgical device undergone through 100 sterilization and cleaning cycle (steam sterilization and alkaline cleaning) and found out marks remained stable and resistant towards corrosion and fading. Acc. to IS 7531:1990.